Clinical SAS Training in Hyderabad
With
100% Placement Support.
Online & Classroom Training | Live Pharma Projects | Internship Support | 3–4 Month Certification
Learn from industry experts at Brolly Academy. Our Clinical SAS Training in Hyderabad gives you hands on exposure to SAS programming, Clinical Data Management (CDM), and CDISC standards. Get job-ready skills and step into pharma and CRO careers with top companies like Parexel, Novartis, Cognizant, and Dr. Reddy’s.
Table of Contents
ToggleClinical SAS Course in Hyderabad
Batch Details
| Trainer Name | Mr. Naveen Kilaru |
| Trainer Experience | 15+ Years |
| Next Batch Date | 16 FEB 2026 |
| Training Modes | Online Live Training (Instructor-Led) |
| Course Duration | 120 Days (120+ Sessions) |
| Call us at | +91 81868 44555 |
| Email Us at | brollyacademy@gmail.com |
| Demo Class Details | ENROLL FOR FREE DEMO CLASS |
Why Choose Brolly Academy for Clinical SAS Training in Hyderabad
500+
Students Placed in Top Pharma & CRO Companies
300+
Google Reviews from Successful Learners
4.8/5
Average Ratings for Training Quality
20,000+
Students Trained Across SAS & Clinical Research
2-3 Month
Month Flexible Course Duration
Modes
Classroom & Online Training Modes
Fee Range
Affordable Fee Range with EMI Options
50+
Live Projects on Real Pharma Data
Why Choose Brolly Academy for Clinical SAS Training in Hyderabad
- 15+ years of Clinical SAS & Pharma expertise.
- Covers Base SAS, Advanced SAS, CDM & CDISC Standards
- Real-time clinical trial projects (5–10 hours hands-on)
- Placement support until successful hiring
- 1-on-1 mentor guidance throughout training
- 200+ students trained, 120+ placements in 4 months
- Certification in Clinical SAS Programming & Data Management
- Online & classroom batches with daily recordings
- Learn Proc Report, Proc Tabulate & SAS Macros
- Hands-on expertise in managing clinical trial data
- Access to interview Q&A bank & curated syllabus
- Live doubt-clearing sessions with certification help
- Practical exposure to Pharmacovigilance applications
- Mock interviews & resume prep for pharma jobs
Clinical SAS Curriculum in Hyderabad
Clinical SAS Course Syllabus
- Overview of Clinical Trials & Clinical Data Management (CDM)
- Regulatory bodies: FDA, ICH-GCP, CDISC, and HIPAA compliance
- Role of SAS in Clinical Research
- Understanding SDTM, ADaM, and TLF standards
- Introduction to SAS environment (SAS Base, SAS EG, SAS Clinical)
- SAS Environment & Libraries
- Data step programming (DATA step, PROC step)
- Importing & exporting clinical data (Excel, CSV, Access, Oracle)
- SAS variables, datasets, and options
- Functions (character, numeric, date/time functions)
- Conditional statements (IF-THEN, DO loops)
- Combining datasets (MERGE, SET, UPDATE, MODIFY)
- PROC SORT, PROC PRINT, PROC FORMAT
- PROC FREQ, PROC MEANS, PROC UNIVARIATE
- PROC REPORT, PROC TABULATE
- PROC TRANSPOSE, PROC SQL for clinical reporting
- Data validation and data cleaning methods
- SDTM (Study Data Tabulation Model) mapping
- ADaM (Analysis Data Model) dataset creation
- Clinical trial raw data to SDTM conversion
- CDISC standards implementation
- Generating subject-level datasets (DM, AE, VS, EX, LB, etc.)
- Managing clinical trial metadata
- PROC SQL for advanced queries and joins
- Macros and macro variables in SAS
- Debugging and error handling in SAS programs
- Automation of clinical reports with SAS Macros
- Efficiency and performance tuning
- Hypothesis testing (t-test, chi-square, ANOVA)
- Survival analysis (PROC LIFETEST, PROC PHREG)
- Regression models (PROC REG, PROC LOGISTIC)
- Mixed models (PROC MIXED, PROC GLM)
- Handling missing data in clinical trials
- Creating safety and efficacy tables
- Generating patient listings and profiles
- Producing figures and charts for clinical studies
- Validating TLF outputs as per submission standards
- Automation of TLF generation with SAS Macros
- FDA submission guidelines (eCTD, define.xml)
- 21 CFR Part 11 compliance
- Data validation & quality checks before submission
- Preparing datasets for regulatory submission (SDTM, ADaM packages)
- End-to-end case study: Raw data → SDTM → ADaM → TLF
- Clinical trial data analysis project
- Mock submission project aligned with FDA standards
- Industry-level assignments
- Frequently asked Clinical SAS interview questions
- Resume building with Clinical SAS projects
- Mock interviews and practical assessments
- Guidance on certifications (SAS Certified Clinical Trials Programmer)
- Placement support in Pharma, CROs, and IT companies
Clinical SAS Training Road Map – Learning Path
At Brolly Academy, we teach Clinical SAS in two ways. You can learn online with live classes from home or join our offline classroom sessions at our Hyderabad campus. Both options cover the same topics, include real projects for practice, and give you full placement support to help you get a job.
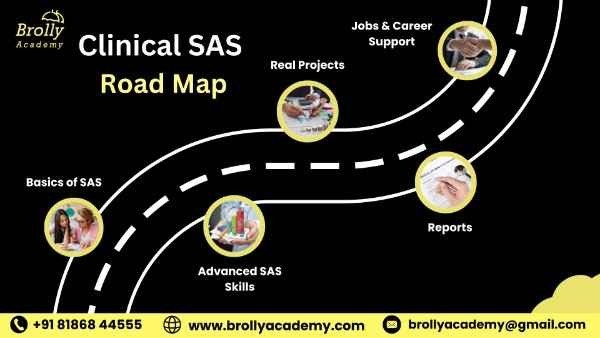
Phase 1 : Learn the Basics of SAS
- Understand how SAS works and how it stores and manages data.
- Write simple SAS programs and create datasets.
- Get an introduction to clinical research and data management.
Phase 2 : Advanced SAS Skills
- Learn advanced tools like PROC REPORT, PROC TABULATE, and SAS Macros.
- Work with real clinical trial data and see how pharma companies use SAS.
- Study CDISC standards (SDTM & ADaM) required for global FDA.
Phase 3: Real Projects & Reports
- Create tables, graphs, and reports used in actual clinical studies.
- Practice with pharma data projects for 5–10 hours.
- Learn how to read and follow a Statistical Analysis Plan (SAP).
Phase 4: Jobs & Career Support
- Prepare with mock interviews, resume building, and interview Q&A.
- Get 100% placement assistance with top pharma and CRO companies.
- Receive certification support to start your career as a Clinical SAS Programmer.
What is Clinical SAS?
- For pharmaceutical and CRO companies, Clinical SAS programming is a key skill used to analyze and report clinical trial data. It plays a key role in drug development, clinical research, and regulatory reporting.
- Definition – Clinical SAS programming, used for analyzing and reporting clinical trial data, is highly valued by pharma and CRO companies.
- Drug Development Role – Clinical SAS is applied between pre-clinical research and post-marketing studies, helping bridge the gap in the drug development process.
- Data Collection – Clinical trial data is captured through Clinical Report Forms (CRF) and stored in secure databases for SAS analysis.
- Animal & Human Trials – As part of clinical research, drugs are first tested on animals and then on human volunteers, with data monitored and recorded using SAS.
- Clinical Data Management (CDM) – Clinical SAS supports CDM by ensuring data is validated, accurate, and clean before statistical analysis.
- Global Opportunities – Certified Clinical SAS professionals find jobs not only in Hyderabad but also in India, USA, and Europe, making it a career with international scope.
- Tools & Reporting – Students in a Clinical SAS Course in Hyderabad learn PROC REPORT, PROC TABULATE, SAS Macros, and other procedures to create tables, graphs, and listings.
- CDISC Standards – Training covers SDTM and ADaM standards, required for FDA and EMA submissions, making it a global skill.
- Real-Time Projects – A Clinical SAS Training Institute provides hands-on projects with pharma datasets for practical learning.
- Statistical Analysis Plan (SAP) – Clinical SAS programmers generate outputs and reports as per the SAP used in clinical trials.
- Pharmacovigilance Use – Widely applied in pharmacovigilance to track and analyze drug side effects during trials.
- Career Scope – Completing a Clinical SAS Course with Placement opens career paths as Clinical SAS Programmer, Statistical Programmer, or Biostatistician with salaries from ₹3.5 LPA to ₹12 LPA.
What is Clinical SAS Used For?
Use Case | Explanation |
Data Collection & Storage | Clinical SAS is used to collect, store, and analyze data generated during clinical research as part of the drug development process. |
Trusted & Confidential | Clinical SAS is preferred because it keeps data safe, secure, and confidential. |
Clear Reporting | Reports and graphical analysis from Clinical SAS are accurate, clear, and easy to process. |
Faster Trials | It speeds up the clinical trial process for researchers by providing quick analysis. |
Big Data Analysis | Helps analyze structured and unstructured data, such as patient insights and trial outcomes. |
Simplified Processing | Allows manipulation, processing, and reporting of data in easy-to-understand graphical forms. |
Two Key Steps in SAS | 1. DATA step – create, manage, and store data of provided 2. PROC step – analyze and represent the data. |
Supports Data Analysis | Enables researchers to try new methods and approaches for analyzing trial data. |
Graphical Outputs | Converts analyzed data into tables, charts, and graphs for clinicians and statisticians to study easily. |

Thinking of Joining a Clinical SAS Course in Hyderabad?
Traditional Training | Brolly Academy Clinical SAS Training |
Mostly theory, very little practical exposure. | Work on real clinical trial datasets and live pharma projects from day one. |
Trainers with only academic background. | Industry experts with 15+ years in pharma & CRO companies. |
Covers only generic SAS programming. | Covers Base SAS, Advanced SAS, Clinical Data Management (CDM), CDISC standards (SDTM & ADaM). |
Limited exposure to tools and case studies. | Hands-on practice with PROC SQL, PROC REPORT, PROC TABULATE, ODS, and Macro Programming. |
No placement support provided. | 100% Placement Assistance with resume prep, mock interviews & referrals. |
Exercises not linked to real clinical work. | Assignments & projects designed around clinical trial reporting. |
No exposure to regulatory standards. | Training aligned with FDA & EMA submission requirements. |
No freelancing/startup guidance. | Guidance for careers in Clinical SAS, Biostatistics, and Pharmacovigilance. |
One-size-fits-all training approach. | Customized learning roadmap for students, freshers, professionals & corporates. |
Benefits of Clinical SAS Training in Hyderabad
Benefits of the Course
1. Jobs in India & Abroad
2. Good Salaries
3. Many Job Options
4. Work From Home Choices
5. Learn by Doing
6. High Demand in Hyderabad
7. Freelance Earning
8. Career Growth
9. Easy Career Switch
Best Clinical SAS Training Institute in Hyderabad
Meet Your Clinical SAS Trainer
INSTRUCTOR
Mr. Naveen Kilaru
Clinical SAS Expert & Pharma Data Analytics Mentor
15+ Years of Experience in Clinical SAS, Pharma & CRO Projects
About the Trainer:
- Mr. Naveen Kilaru is a highly experienced Clinical SAS Trainer in Hyderabad with over 15 years of expertise in SAS programming, clinical research, and data management.
- He has worked with top CROs (Clinical Research Organizations) and pharma companies on large-scale clinical trials, preparing datasets and reports for global submissions.
- He is skilled in handling clinical data, making reports, and creating tables using SAS tools. He knows how to write simple programs to save time, follow international rules for clinical data, and prepare safety reports about medicines.
- At Brolly Academy Hyderabad, he has successfully trained 2,000+ students and professionals, helping them build careers as Clinical SAS Programmers, Statistical Programmers, and Biostatisticians.
- Mr. Naveen combines real-world pharma projects with step-by-step teaching methods, ensuring students gain hands-on SAS experience that is directly useful in the job market.
- His training covers clinical trial case studies, SAP (Statistical Analysis Plan) reporting, and FDA/EMA submission requirements, making learners industry-ready.
- With his proven record of placement support, many of his students are now working in Hyderabad’s leading pharma and IT companies like Parexel, Novartis, Cognizant, and Dr. Reddy’s.
Skills Developed Post Clinical SAS Classes in Hyderabad
At Brolly Academy, our Clinical SAS Training in Hyderabad online and offline Classes helps you gain practical skills step by step.
- 1. Clinical Research Skills You will learn how clinical research works and how SAS is used to study the data collected during drug trials.
- 2. Clinical SAS Software Skills You will get hands-on practice with SAS software to perform real clinical tasks just like professionals in pharma companies.
- 3. Clinical SAS Architecture You will understand the structure of Clinical SAS – how it is built, how it stores data, and how different parts of it work together.
- 5. Output Delivery System (ODS) You will gain experience in using ODS to create reports in different formats like tables, charts, and graphs.
- 6. SAS Procedures You will practice using many SAS procedures (PROCs) that make it easy to analyze and prepare clinical trial data.
- 7. PROC SQL You will learn PROC SQL, a powerful tool to quickly search, merge, and manage large volumes of clinical trial data
- 8. CDISC Principles You will study CDISC standards like SDTM and ADaM, which are global rules followed for drug approvals by agencies like FDA.
- 9. Data Management You will develop the ability to manage and transform trial data, making it ready for reports and regulatory submission.
Clinical SAS Capstone Projects in Our Hyderabad
Clinical SAS Projects Covered
1. Clinical Trial Data Management Project
Work on real clinical trial datasets to clean, validate, and structure data. Learn to handle CRFs (Clinical Report Forms) and prepare datasets for further analysis.
2. Base SAS & Data Step Programming Project
Practice creating and managing SAS libraries, data sets, and variables. Apply Data Step programming to manipulate raw trial data.
3. PROC SQL & Reporting Project
Use PROC SQL for querying and merging clinical trial datasets. Generate reports with PROC REPORT, PROC TABULATE, and ODS for clear data visualization.
4. CDISC Standards Project
Implement SDTM and ADaM standards to format clinical trial data as required by FDA and EMA submissions.
5. Statistical Analysis Plan (SAP) Project
Create tables, listings, and graphs (TLGs) as per the SAP guidelines. Learn how to study adverse events and measure patient outcomes effectively.
6. Macro Programming & Automation Project
Develop SAS macros to automate repetitive tasks like generating clinical reports, saving time, and reducing errors in large datasets.
Clinical SAS Training Fees & Training Modes in Hyderabad
Clinical SAS Course Fee & Offerings
Video Recording
Rs 15000 7999
- Lifetime video access
- Basic to advanced level training
- 80+ recorded classes
- Capstone project
- Resume & interview support
- 100% placement assistance
- WhatsApp group access
Class Room Training
Rs 25,000 19,999
- 3.5 months structured training
- Faculty with 15+ years of Clinical SAS industry experience
- Real-time industry projects with pharma trial datasets
- One-on-one mentorship
- Weekly & monthly mock interviews
- Resume & interview guidance
- Soft skills & aptitude training
- Dedicated placement officer
- Commute support (Hyderabad batches)
- WhatsApp support + group access
Online Course
Rs 18000 14,999
- Live interactive classes with flexible timings
- 3–4 months course duration
- Daily class recordings for revision
- Real-time project practice from Day 1
- Weekly mock interviews
- One-on-one doubt-clearing sessions
- 50+ sample resources and interview Q&As
- WhatsApp group access
Our Placement Process for Clinical SAS Coaching in Hyderabad
Online, Offline & Institute-Based Training

Resume Building

Placement Training

Interview Questions

Internships Under Experts

Realtime Live Projects

Get Offer Letter

Scheduling Interviews

Mock Interviews

Personality Development

Aptitude Preparation
- 1. Resume Building We help you prepare a professional resume that highlights your SAS and clinical trial project skills.
- 2. Placement Training Our trainers guide you on the key job skills required in pharma and CRO companies.
- 3. Interview Questions Practice with real Clinical SAS interview questions asked by top employers in Hyderabad.
- 4. Internships with Experts Work on live pharma datasets and internships (available for both online & offline students).
- 5. Real-Time Projects Gain hands-on practice with clinical trial data in Base SAS, Advanced SAS, PROC SQL, and CDISC standards.
- 6. Aptitude Preparation Get trained in problem-solving and logical reasoning for job selection tests.
- 7. Personality Development We help you improve communication, English speaking, and confidence for interviews.
- 8. Mock Interviews Participate in mock interviews (online & offline) and receive feedback from experienced experts.
- 9. Scheduling Interviews We connect you with hiring companies in Hyderabad like Parexel, Novartis, Cognizant, and Dr. Reddy’s.
- 10. Get Offer Letter We support you till you receive your job offer letter and start working as a Clinical SAS Programmer.
Clinical SAS Student Community in Hyderabad
Student Community

Learning & Collaboration
Work with fellow students on real clinical trial datasets, share coding strategies, and solve Clinical SAS challenges together.

Access to Resources and Tools
Get access to SAS software, sample pharma datasets, CDISC guidelines (SDTM & ADaM), and recorded study sessions for revision.

Networking Opportunities
Connect with CRO recruiters, pharma professionals, and alumni working in companies like Parexel, Novartis, Cognizant, and Dr. Reddy’s in Hyderabad.

Mentorship from Industry Professionals
Learn directly from Clinical SAS experts with 15+ years of experience in pharma and CRO projects.

Job Support and Career Development
Receive continuous guidance with placement referrals, mock interviews, freelancing opportunities, and career-building sessions to ensure long-term success in Clinical SAS.
Who Can Learn Clinical SAS Training in Hyderabad?
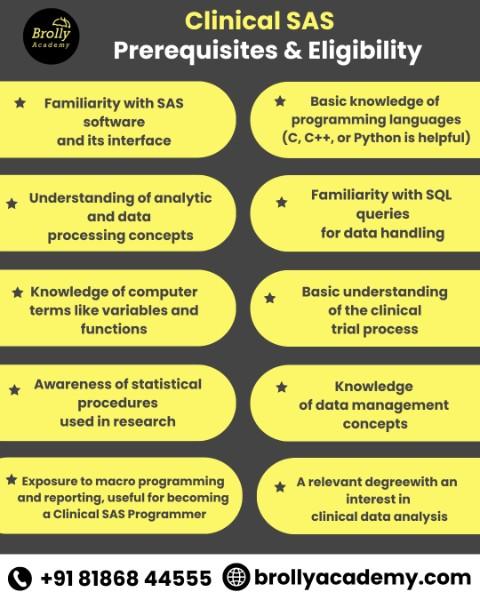
Pre-requisites & Eligibility
- Familiarity with SAS software and its interface.
- Basic knowledge of programming languages (C, C++, or Python is helpful).
- Understanding of analytics and data processing concepts.
- Familiarity with SQL queries for data handling.
- Knowledge of computer terms like variables and functions.
- Basic understanding of the clinical trial process.
- Awareness of statistical procedures used in research.
- Knowledge of data management concepts.
- Exposure to macro programming and reporting, useful for becoming a Clinical SAS Programmer.
- A relevant degree (life sciences, pharmacy, statistics, computer science, or related fields) with an interest in clinical data analysis.
Who Should Learn Clinical SAS Training in Hyderabad?
Eligibility to Join Clinical SAS Course

Who Can Join?
- Life Science & Pharmacy Students – B.Pharm, M.Pharm, Biotechnology, Microbiology, Nursing, and Life Science graduates who want to enter the clinical research and SAS programming field.
- Statistics & Computer Science Graduates – Students from Statistics, Mathematics, Computer Science, or Data Analytics backgrounds who want to specialize in SAS programming and data reporting.
- Freshers Looking for Jobs – Students seeking Clinical SAS fresher jobs in Hyderabad with placement assistance.
- Working Professionals – IT and healthcare professionals who want to switch careers into Clinical SAS, Statistical Programming, or Clinical Data Management (CDM).
- Pharma & CRO Employees – Employees from pharma companies and Clinical Research Organizations (CROs) who want to upgrade skills in SAS and CDISC standards.
- Freelancers & Consultants – Individuals aiming to offer freelance SAS programming, data management, and statistical reporting services to global clients.
- Students Without Coding Background – Anyone with basic computer knowledge can start, as Clinical SAS training is beginner-friendly.
Career Opportunities after Clinical SAS Training in Hyderabad
Career Opportunities

Career Opportunities
- Clinical Data Analyst Analyze patient data from clinical trials, apply statistical methods, generate insights, and support decision-making for drug development and safety monitoring.
- Clinical Data Manager Oversee the collection, validation, and storage of clinical data, ensuring accuracy and compliance with regulatory requirements such as FDA and EMA.
- Clinical Data Associate Assist in data entry, query management, and data validation processes for ongoing clinical trials. Ensure quality and completeness of trial data.
- Clinical SAS Consultant Provide specialized consulting to pharma companies, CROs, and healthcare organizations, focusing on SAS programming, data reporting, and regulatory compliance.
Clinical SAS Jobs in Hyderabad – Salary Range
Job Role | Average Salary (Hyderabad) | Salary Range (Freshers to Experienced) |
₹5.0 LPA | ₹3.6 LPA – ₹5.7 LPA | |
Senior Clinical Data SAS Programmer | ₹12.6 LPA | ₹11.7 LPA – ₹14.0 LPA |
Clinical SAS Programmer (India Average) | ₹4 – 10 LPA | ₹3.0 LPA – ₹15.0 LPA |
Clinical Data Analyst | ₹5.5 LPA | ₹3.5 LPA – ₹8.0 LPA |
Clinical Data Manager | ₹7.0 LPA | ₹4.5 LPA – ₹12.0 LPA |
Clinical Data Associate | ₹4.0 LPA | ₹2.8 LPA – ₹6.5 LPA |
Clinical SAS Consultant | ₹10.0 LPA | ₹6.0 LPA – ₹18.0 LPA |
Hiring Companies for Clinical SAS Jobs in Hyderabad






Our Achievements as the Best Clinical SAS Training Institute in Hyderabad
Our Achievements
Completed 80+ Clinical SAS Batches
15+ Years of Industry Training Experience
1000+ Students & Professionals Trained
700+ Successful Placements in Pharma & CROs
Clinical SAS Certifications in Hyderabad Training Program
After completing the Clinical SAS Training in Hyderabad with Brolly Academy, you will receive industry-recognized certifications that validate your expertise in SAS programming, Clinical Data Management, and CDISC standards. These certifications are accepted by pharma companies, CROs, and healthcare organizations worldwide.

Clinical SAS Certifications You Will Receive
- Clinical SAS Course Completion Certificate – Validates your knowledge of Base SAS & Advanced SAS programming.
- Clinical Data Management Certification – Focused on data handling, cleaning, and validation in clinical trials.
- CDISC Standards Certification (SDTM & ADaM) – Required for regulatory submissions like FDA, EMA.
- Clinical SAS Project Completion Certificate – Earned by working on real-time pharma datasets and projects.
- Placement Readiness Certification – Covers mock interviews, resume building, and job preparation.
Clinical SAS Market Trends & Job Growth in Hyderabad 2026
Market Trends
- Global Clinical Trials Growth – The global clinical trials market is expected to grow from USD 64.9 billion in 2026 to USD 104.4 billion by 2032, with a steady annual growth rate of 6.8%.
- India’s Clinical Data Analytics Boom – India’s clinical data analytics market is projected to reach USD 7 billion by 2026, growing at an impressive 22% CAGR, creating huge demand for Clinical SAS programmers.
- Hyderabad: Pharma & CRO Hub – Known as India’s Genome Valley, Hyderabad is home to 250+ pharma & life sciences companies including Dr. Reddy’s, Novartis, Parexel, ICON, and IQVIA, driving SAS hiring.
- Fresher Hiring Momentum – Around 60% of Clinical SAS jobs in Hyderabad are open to freshers, with salaries starting at ₹3.5 LPA and experienced roles reaching ₹18 LPA.
- Job Growth in Hyderabad – Hyderabad remains one of the fastest-growing pharma & clinical research job markets, with thousands of SAS roles expected by 2027.
- Nationwide Demand – India ranks in the top 5 global CRO destinations, with Hyderabad, Bangalore, and Pune leading in SAS programming and clinical data management hiring.

Clinical SAS Training in Hyderabad
FAQS
1. What is Clinical SAS Training?
2. Who can join Clinical SAS Training?
3. Is Clinical SAS easy to learn for beginners?
4. What are the requirements to join Clinical SAS?
5. Can I learn Clinical SAS online?
6. How long is the Clinical SAS Course in Hyderabad?
7. What are the Clinical SAS course fees in Hyderabad?
8. How much time does it take to master Clinical SAS?
9. How long is the SAS certification training?
10. How much does SAS certification cost in India?
11. Is Clinical SAS in demand in Hyderabad?
Yes. Hyderabad is a pharma hub with 100+ CROs and MNCs hiring SAS programmers every year.
12. What is the future of Clinical SAS?
13. Can freshers get jobs in Clinical SAS?
14. How can I get a job in Clinical SAS?
15. Which companies hire Clinical SAS professionals in Hyderabad?
16. What is the salary of a Clinical SAS Programmer in Hyderabad?
17. How much do Clinical SAS Programmers earn at TCS?
18. What is the salary at Cognizant for Clinical SAS jobs?
19. What is the highest salary for Clinical SAS in Hyderabad?
20. How much can I earn after 3 years in Clinical SAS?
21. Do you provide placement support?
22. Can I attend a demo class before joining?
23. What if I miss a class during the training?
24. Do I get a certificate after completing the course?
25. Do you offer both classroom and online training?
26. What is the full form of SAS?
27. Is SAS software free?
28. Can I learn SAS without a background in life sciences?
29. Does SAS certification expire?
No, once you get certified, it is valid for life.
30. Is SAS a good career option in India?
Other Relevant Courses
Got more questions?
Talk to Our Team Directly
Contact us and our academic councellor will get in touch with you shortly.











